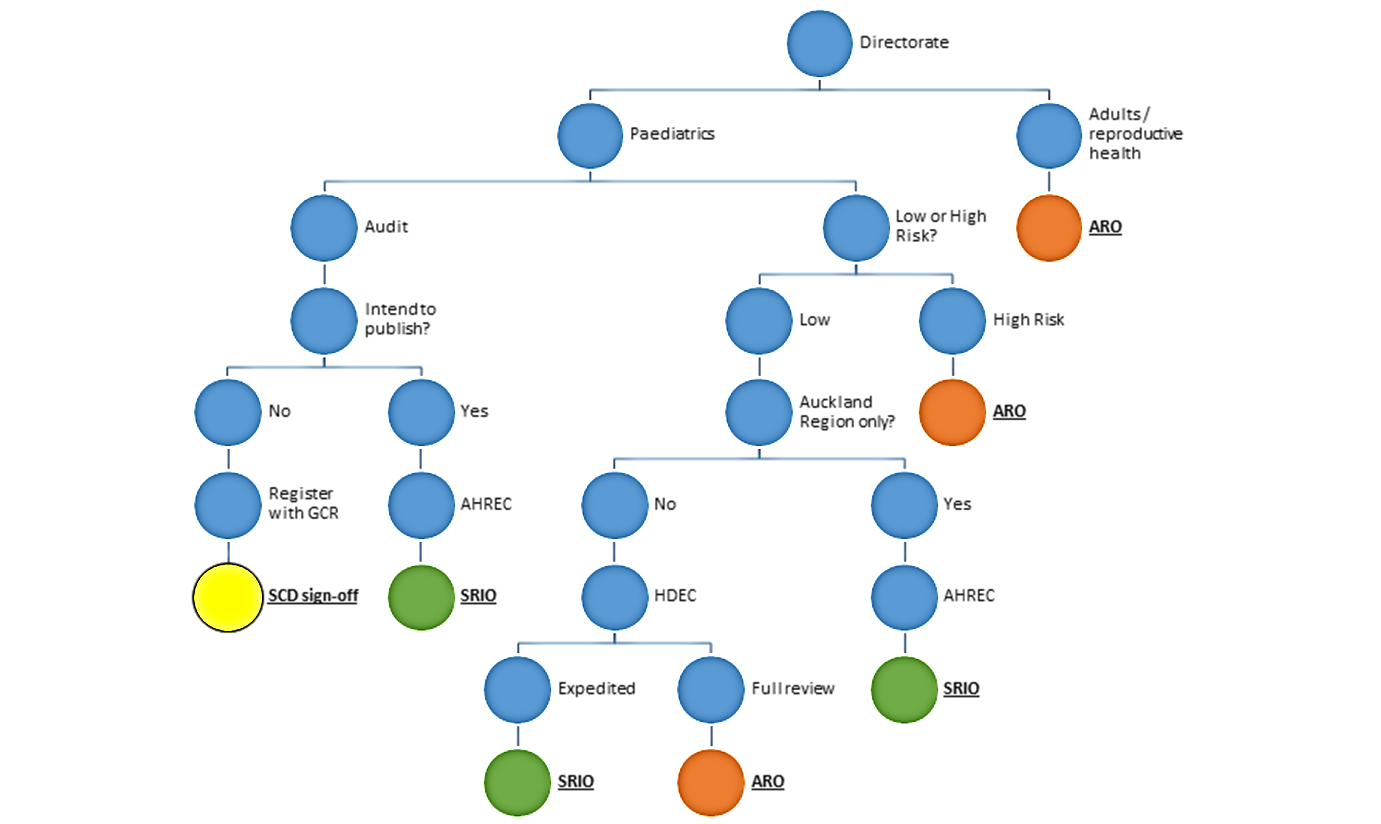
Research approval pathway
Starship Research & Innovation Office (SRIO) and ADHB Research Office (ARO) responsibilities
1. Research proposal evaluation
Assign low risk (retrospective, prospective observational, case control, cohort) or high risk (intervention, commercial study, prospective evaluation). Ethics may be given for low risk studies where the study meets AHREC criteria or HDEC out of scope confirmation, or expedited review.
This task can be undertaken by either office.
2. Register the study
All high risk studies must be registered by the ARO and assigned a unique study number (A+ number)
SRIO will register all low risk studies with a unique A+ number
3. Assign locality review and approval
Minimal risk projects – this task can be undertaken by either office.
All other projects – this task is undertaken by the ARO for RRC review.
4. Obtain Māori Review and approval
This task is coordinated by the either office.
5. Legal and contract review
All contracts and legal documents must be approved by ADHB legal team. Contract neogotiation with funders/sponsors together with mandatory approvals (ethics, Māori, SCOTT) and RRC approval is required for final sign off before contract is signed by A+ Trust.
These tasks can be performed by either office. The Director SS Child Health or CMO respectively signs the blue sheet prior to submission for contract signing.